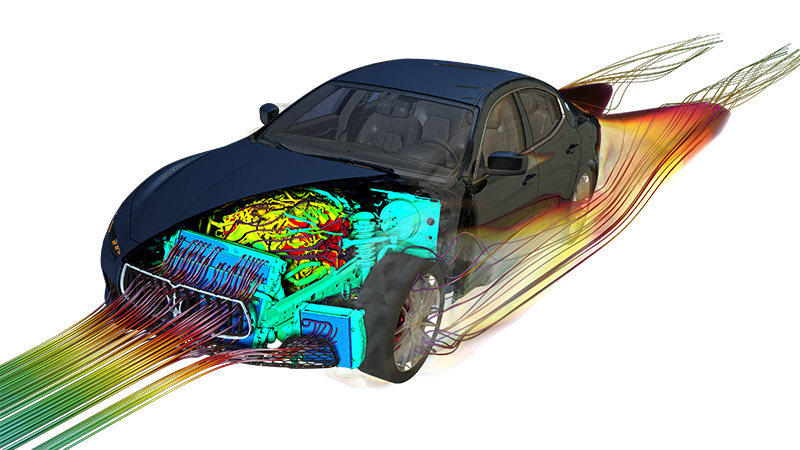
Thermal Management
By DarthVader
Date: 2021-11-14
Topic: 81 see comments
Post views: 6601
Taking care to balance thermal energy inputs and outputs is an important part of engineering systems.
If thermal energy is generated within a device, then its temperature will rise until it is able to pass on its thermal energy at the same rate at which it is generated or else until the heat generation stops. The temperature rise of an object associated with a given amount depends on the objects mass and its overall specific heat capacity.
The forced flow of air or water is often used to keep devices cool. The flow of any coolent needs to be sufficient to have the capacity to carry heat away at the same rate as it is generated.
Three Types of Heat Transfer:
- Conduction
- Convection
- Radiation
Specific Heat Capacity
The specific heat capacity of a substance is the heat energy required in joules to raise the temperature of 1 kg of that substance by 1 k (1 °C)
Example:
Specific Heat Capacity of air = 1000 c (J kg-1 °C-1)
This means it takes 1000 J of energy to raise the temperature of 1 kg of air by 1 °C (k)
| Comments | Creator | Date | ID |
|---|