Anode and Cathode Overview
By DarthVader
Date: 2024-11-15
Topic: 212 see comments
Post views: 33
Rules:
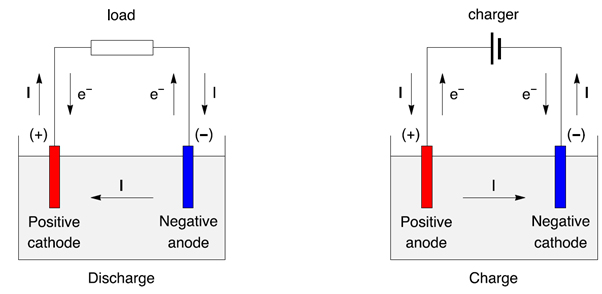
- Electrons always flow from the anode to the cathode always no matter what :]
- The anode is always where oxidation occurs (Loss of electrons).
- The cathode is always where reduction occurs (Gain of electrons).
Key points:
Although the flow of electrons always stays the same, the polarity can change for each electrode.
1. Power producing devices i.e. batteries: [energy source]
The anode is negative because electrons are generated here (source), and sent out into the circuit.
2. Power consuming devices i.e. electrolytic cells: [energy sink]
The anode is positive because it needs to be connected to a power source, and receive electrons in order to work.
3. Diodes:
Anode is positive
| Comments | Creator | Date | ID |
|---|