Heating, Cooling And Changes Of State
By DarthVader
Date: 2022-06-27
Topic: 138 see comments
Post views: 1212
Heating, Cooling And Changes Of State
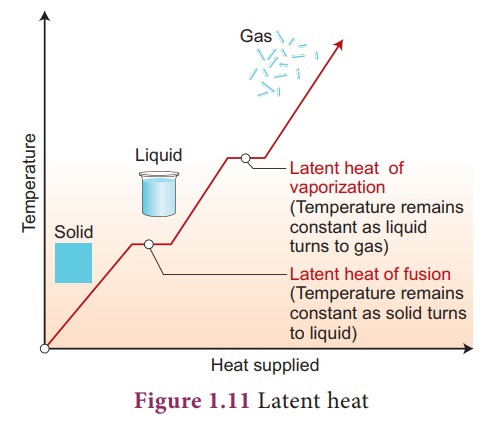
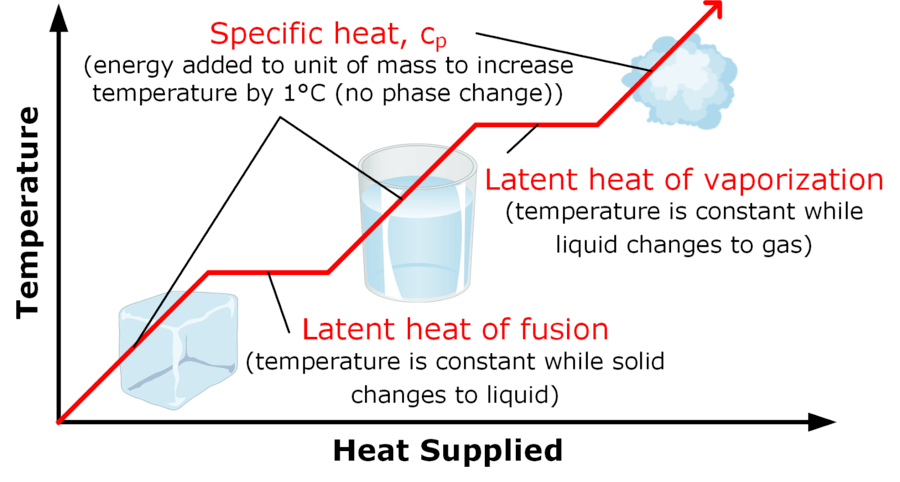
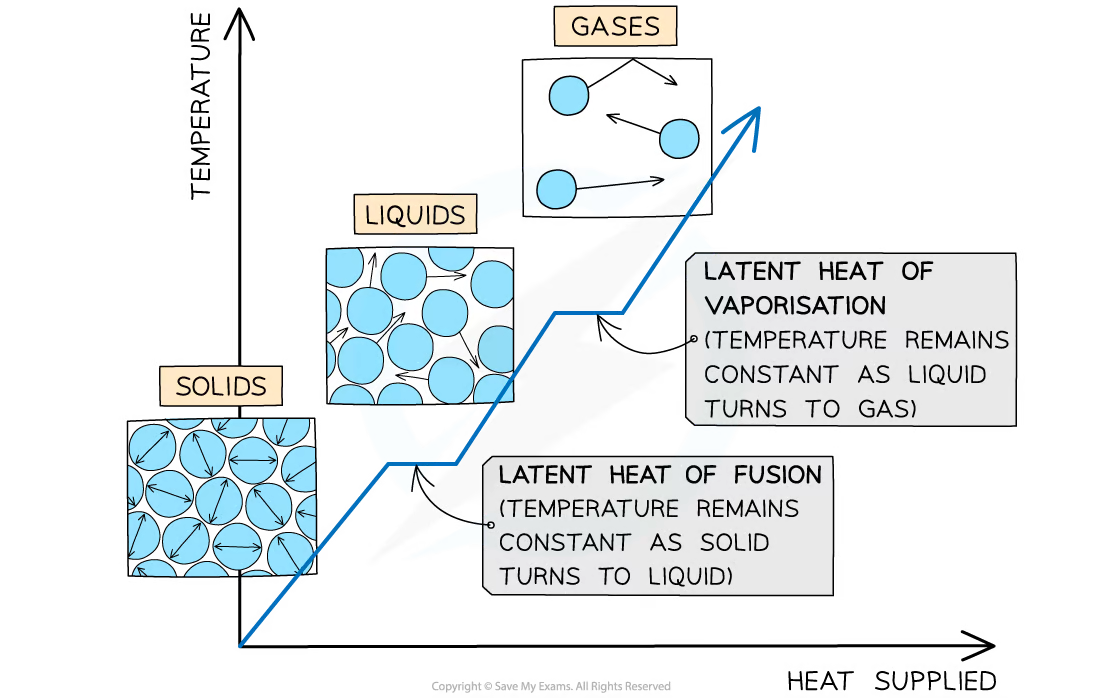
Taking casting as an example of a material cooling, freezing (solidifying), and then cooling to room temperature, the total heat energy released can be calculated by working each of the three steps individually and then adding them up. Two equations are needed, the specific heat capacity equation and the specific latent heat equation.
Specific heat capacity:
ΔQ = mcΔT
Specific latent heat of fusion: (heat energy released from material freezing)
ΔQ = mLf
Specific latent heat of vaporisation: (heat energy absorbed by material melting)
ΔQ = mLv
where:
ΔQ = heat energy (kJ)
m = mass (kg)
L = Specific latent heat (kJ kg-1)
In order to calculate the heat energy released or absorbed by a material which also undergoes a ‘change of state’ from solid, to liquid, or to gas, you first need to know three properties of the material:
- Melting temperature, Tm (°C)
- Specific latent heat of fusion (or vaporisation), Lf, or: Lv, (kJ kg-1)
- Specific heat capacity, c (kJ kg-1 °C-1)
Heating Equations
Electrical Heating:
MC(T2 - T1) = IVt
where:
M = mass
C = specific heat capacity
T2 = final temperature
T1 = initial temperature
I = current
V = voltage
t = time
This equation is used to work out the temperature change of a substance if heating is provided from an electrical source such as an electric furnace etc.
Fuel Heating:
MC(T2 - T1) = ERt
M = mass
C = specific heat capacity
T2 = final temperature
T1 = initial temperature
E = energy from fuel per kg
R = rate of fuel burned in kg per second
t = time
This equation is used to work out the temperature change of a substance if fuel is burned to provide heating. Fuel used might be coal, oil or wood etc.
| Comments | Creator | Date | ID |
|---|